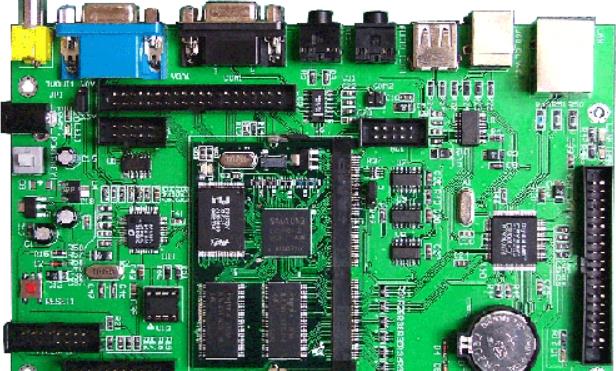
PCBA Medical Equipment Responds to Coronavirus
As we all know, the demand for medical ventilators and other "life sustaining" equipment is growing. With the sharp increase in the number of patients in hospitals, the supply chain of medical equipment has become strained
During the pandemic, the US Food and Drug Administration (FDA) tried to increase the production and supply of ventilators, accessories and other respiratory equipment. This is the latest information on the efforts of the US Food and Drug Administration and some important suggestions on managing the supply chain of medical devices in uncertain times.
During the 2019 coronavirus disease scare, many enterprises, including manufacturers, were temporarily suspended However, the manufacturer PCBA medical powered electronic equipment still works
As the US Food and Drug Administration points out, there is no alternative unless demand calms down.
Circuit board
"With the improvement of the US Food and Drug Administration (production and supply), medical device manufacturers can more easily make changes to existing products, such as changing suppliers or materials, to help solve the current manufacturing restrictions or supply shortages. Other manufacturers (such as automobile manufacturers) can more easily readjust the use of their production lines to help increase supply.
Maintain the delivery cycle of PCBA medical equipment supply chain
Due to the expected shortage of products, delivery time is more important than ever.
Generally speaking, every time a manufacturer modifies a ventilator, it will trigger a pre marketing review by the US Food and Drug Administration. This will increase the time it takes to transport these devices to the patient bed.
In order to reduce the regulatory burden on manufacturers, the US Food and Drug Administration now uses its discretion to decide whether to implement review requirements for certain modifications of medical devices.
The agency encourages U.S. and international electronic manufacturers to consider seeking emergency use authorization, which will enable them to distribute respirators in the United States. This includes American manufacturers that have already produced medical devices but can increase their output.
What PCBA supply chain is needed
Since electronic medical device manufacturers manufacture products for life sustaining devices, they are still in business, and they provide everything to OEMs as soon as possible.
Working with manufacturers who understand the risks and challenges of medical electronics design is more important than ever. The manufacturer shall have:
·Design engineering teams familiar with mission critical applications
·Production facilities capable of building, testing and tracking components
·Comprehensively understand ISO 13485 requirements and years of experience
Level 3 electronic experience
PCBA electronic medical equipment belongs to IPC 3 electronic products. They are considered as "critical tasks", and equipment failure may endanger human life.
Cooperation with PCBA manufacturers with expertise in three types of projects can shorten the entry speed and increase the possibility of design to meet your strict safety requirements. All employees of contractors involved in the project (not just some employees) shall have Level 3 experience.
ISO 13485 certification and other training
ISO 13485 medical certification is a seal developed by the contract manufacturer of electronic products and approved as a quality control system. It ensures that contractors produce safe, reliable and efficient PCB boards and components
PCB designers shall be trained in all relevant standards:
·Surface Mount Technology and through-hole technology production: J-STD-001 and IPC-610
·Harness production: IPC-620
·PCB rework and repair: IPC-7711
The above is the explanation given by the editor of pcb circuit board company.
If you want to know more about PCBA, you can go to our company's home page to learn about it.
In addition, our company also sells various circuit boards,
High Frequency Circuit Board and SMT chip are waiting for your presence again.